top of page

Post-doctoral fellow

21. Lewis Acid-Catalyzed Unusual (4+3) Annulation of para-Quinone Methides with Bicyclobutanes: Access to Oxabicyclo [4.1.1]octanes.
Deswal, S.; Guin, A.; Biju, A. T.*
Angew. Chem. Int. Ed. 2024, 63, e202408610. ChemRxiv. 2024, Preprint.

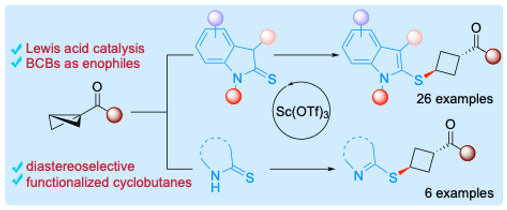
20. Lewis Acid-Catalyzed Diastereoselective Ene Reaction of Thioindolinones/Thiolactams with Bicyclobutanes.
Guin, A.*; Deswal, S.; Biju, A. T.*
Chem. Sci. 2024, 15, 12473-12479. ChemRxiv. 2023, Preprint.

19. Lewis Acid-Catalyzed One-Pot Thioalkenylation of Donor-Acceptor Cyclopropanes Using in situ Generated Dithiocarbamates and Propiolates.
Harikumar, S.; Kandy, L. T. K.; Guin, A.*; Biju, A. T.*
Org. Biomol. Chem. 2024, 22, 1834-1838. Invited to the special issue celebrating the 100th birthday of Prof. Sukh Dev.

















18. Stereoselective Alder-ene Reactions of Bicyclo[1.1.0]butanes: Facile Synthesis of Cyclopropyl- and Aryl-substituted Cyclobutenes.
Dasgupta, A.; Bhattacharjee, S.; Tong, Z.; Guin, A.; McNamee, R.; Christensen, K.; Biju, A. T.*; Anderson, E.*
J. Am. Chem. Soc. 2024, 146, 1196-1203. ChemRxiv. 2023, Preprint.
17. Lewis Acid-Catalyzed Diastereoselective Carbofunctionalization of Bicyclobutanes Employing Naphthols.
Guin, A.; Bhattacharjee, S.; Harariya, M. S.; Biju, A. T.*
Chem. Sci. 2023, 14, 6585-6591.
16. Benzotriazole-Triggered Three-Component Lewis Acid-Catalyzed Ring-Opening 1,3-Aminofunctionalization of Donor–Acceptor Cyclopropanes.
Deswal, S.; Guin, A.; Biju, A. T.*
Org. Lett. 2023, 25, 1643-1648.
15. Ring-Opening 1,3-Difunctionalization of Donor-Acceptor Cyclopropanes.
Guin, A.; Biju, A. T.*
In "Donor-Acceptor Cyclopropanes in Organic Synthesis", Chapter 6, Editors: P. Banerjee and A. T. Biju; Wiley-VCH. 2023, ISBN: 978-3-527-349876, In Press.

14. Synthesis of Trisubstituted Oxazoles via Aryne Induced [2,3] Sigmatropic Rearrangement-Annulation Cascade.
Gaykar, R. N.; Deswal, S.; Guin, A.; Bhattacharjee, S.; Biju, A. T.*
Org. Lett. 2022, 24, 4145-4150.
13. Ring-Opening 1,3-Carbothiolation of Donor−Acceptor Cyclopropanes Using Alkyl Halides and In Situ Generated Dithiocarbamates.
Guin, A.; Deswal, S.; Biju, A. T.*
J. Org. Chem. 2022, 87, 6504-6513.
12. Molecular Rearrangements.
Guin, A.; Deswal, S.; Biju, A. T.*
In "Comprehensive Aryne Synthetic Chemistry", Chapter 3-4, Editor: H. Yoshida; Elsevier, 2021, Pages 223-266.
11. Three-Component, Diastereoselective [6+3] Annulation of Tropone, Imino Esters and Arynes.
Guin, A.; Gaykar, R. N.; Deswal, S.; Biju, A. T.*
Org. Lett. 2021, 23, 7456-7461.
10. Transition-Metal-Free C2-Functionalization of Pyridines via Aryne Three-Component Coupling.
Guin, A.; Bhattacharjee, S.; Biju, A. T.*
Chem. Eur. J. 2021, 27, 13864-13869.
Selected as a “Hot Paper” by the Editors
9. An Umpolung Oxa-[2,3] Sigmatropic Rearrangement Employing Arynes for the Synthesis of Functionalized Enol Ethers.
Gaykar, R. N.; George, M.; Guin, A.; Bhattacharjee, S.; Biju, A. T.*
Org. Lett. 2021, 23, 3447-3452.
8. Hetarynes, Cycloalkynes and Related Intermediates.
Guin, A.; Bhattacherjee, S.; Biju, A. T.*
In "Modern Aryne Chemistry", Chapter 9, Editor: A. T. Biju; Wiley-VCH. 2021, ISBN: 978-3-527-34646-2, pages 359-406.
5. Lewis Acid-Catalyzed Ring-Opening 1,3-Aminothiolation of Donor-Acceptor Cyclopropanes Using Sulfenamides.
Guin, A.; Rathod, T.; Gaykar, R. N.; Roy, T.; Biju, A. T.*
Org. Lett. 2020, 22, 2276-2280.
4. Three-Component Aminoselenation of Arynes.
Gaykar, R. N.; Guin, A.; Bhattacharjee, S.; Biju, A. T.*
Org. Lett. 2019, 21, 9613-9617.

1. Formal [4+2] benzannulation of 2-alkenyl indoles with aldehydes: a route to structurally diverse carbazoles and bis-carbazoles.
A. Banerjee, A. Guin, S. Saha, A. Mondal and M. S. Maji*
Org. Biomol. Chem. 2019, 17, 1822-1826.
bottom of page











.png)
.png)





.png)